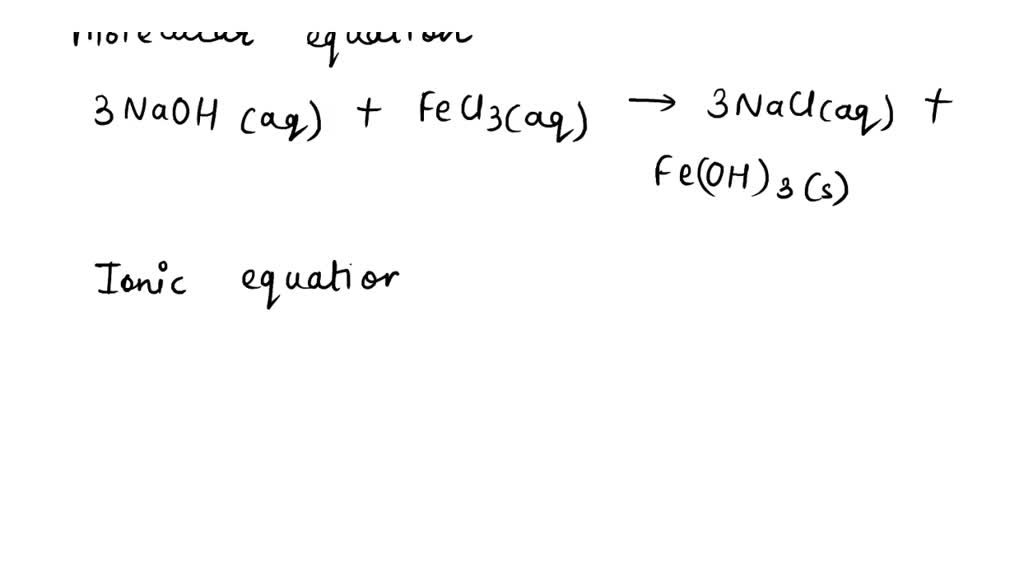Iron Ii Chloride Reaction With Water . common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. in this video we will describe the equation fecl2 + h2o and write what happens. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. Fe + 2hcl → fecl 2 + h 2; commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. for example, concrete pillars have iron rebar in them for strength. predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. Iron metal reacts with air, water vapor,. However, salt water can quickly react with the iron to form iron (ii) chloride. The chemical equation for a reaction in solution can be written in three ways.
from www.numerade.com
an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. Iron metal reacts with air, water vapor,. common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. in this video we will describe the equation fecl2 + h2o and write what happens. However, salt water can quickly react with the iron to form iron (ii) chloride. The chemical equation for a reaction in solution can be written in three ways. for example, concrete pillars have iron rebar in them for strength. Fe + 2hcl → fecl 2 + h 2;
SOLVED 14. What is the net ionic equation for the reaction of aqueous
Iron Ii Chloride Reaction With Water common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. However, salt water can quickly react with the iron to form iron (ii) chloride. Fe + 2hcl → fecl 2 + h 2; Iron metal reacts with air, water vapor,. The chemical equation for a reaction in solution can be written in three ways. in this video we will describe the equation fecl2 + h2o and write what happens. predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. for example, concrete pillars have iron rebar in them for strength. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride.
From www.numerade.com
SOLVED Write a balanced molecular equation for the reaction that Iron Ii Chloride Reaction With Water However, salt water can quickly react with the iron to form iron (ii) chloride. for example, concrete pillars have iron rebar in them for strength. The chemical equation for a reaction in solution can be written in three ways. common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. in this. Iron Ii Chloride Reaction With Water.
From www.alamy.com
Iron reacting with hydrochloric acid. Image 1 of 4. Iron (Fe) reacts Iron Ii Chloride Reaction With Water predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. Iron metal reacts with air, water vapor,. common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride.. Iron Ii Chloride Reaction With Water.
From www.numerade.com
SOLVED The compound iron(II) chloride is a strong electrolyte. Write Iron Ii Chloride Reaction With Water in this video we will describe the equation fecl2 + h2o and write what happens. predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. However, salt water can quickly react with the iron to form iron (ii) chloride. an aqueous solution of strontium hydroxide is added to. Iron Ii Chloride Reaction With Water.
From www.numerade.com
SOLVED Part A Enter a balanced chemical equation for the reaction of Iron Ii Chloride Reaction With Water predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. in this video we will describe the equation fecl2 + h2o and write what happens. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. for example, concrete pillars have iron rebar. Iron Ii Chloride Reaction With Water.
From www.chegg.com
Solved Reaction 14 Aqueous iron(II) chloride + aqueous Iron Ii Chloride Reaction With Water common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Iron metal reacts with air, water vapor,. Fe + 2hcl → fecl 2 + h 2; commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. in this video we will describe the equation fecl2 + h2o and write. Iron Ii Chloride Reaction With Water.
From www.shutterstock.com
Ironii Chloride Chemistry Redox Reaction Stock Vector (Royalty Free Iron Ii Chloride Reaction With Water common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. The chemical equation for a reaction in solution can be written in three ways. Iron metal reacts with air, water vapor,. commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. predicting the solubility of ionic compounds in water. Iron Ii Chloride Reaction With Water.
From www.numerade.com
SOLVED Iron reacts with hydrochloric acid to produce iron(II) chloride Iron Ii Chloride Reaction With Water common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. The chemical equation for a reaction in solution can be written in three ways. Fe + 2hcl → fecl 2 + h 2; for example, concrete pillars have iron rebar in them for strength. predicting the solubility of ionic compounds in. Iron Ii Chloride Reaction With Water.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Iron Ii Chloride Reaction With Water However, salt water can quickly react with the iron to form iron (ii) chloride. The chemical equation for a reaction in solution can be written in three ways. predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. Iron metal reacts with air, water vapor,. an aqueous solution of. Iron Ii Chloride Reaction With Water.
From www.numerade.com
SOLVED Write the chemical equation for each reaction described below Iron Ii Chloride Reaction With Water Iron metal reacts with air, water vapor,. common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. Fe + 2hcl → fecl 2 + h 2; for example, concrete pillars have iron rebar in them for strength. predicting the solubility of ionic compounds in water can give insight into whether or. Iron Ii Chloride Reaction With Water.
From www.youtube.com
Iron and Copper II Chloride Reaction YouTube Iron Ii Chloride Reaction With Water common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. for example, concrete pillars have iron rebar in them for strength. in this video we will describe the equation fecl2 +. Iron Ii Chloride Reaction With Water.
From oneclass.com
OneClass Consider the reaction when aqueous solutions of iron(II Iron Ii Chloride Reaction With Water commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. Iron metal reacts with air, water vapor,. in this video we will describe the equation fecl2 + h2o and write what happens. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. for example, concrete pillars have iron rebar. Iron Ii Chloride Reaction With Water.
From www.youtube.com
Lewis Structure of Iron (II) Chloride, FeCl2 YouTube Iron Ii Chloride Reaction With Water an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. Fe + 2hcl → fecl 2 + h 2; in this video we will describe the equation fecl2 + h2o and write what happens. Iron metal reacts with air, water vapor,. common chemical reactions of iron relate to iron in either a. Iron Ii Chloride Reaction With Water.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick Iron Ii Chloride Reaction With Water in this video we will describe the equation fecl2 + h2o and write what happens. for example, concrete pillars have iron rebar in them for strength. Iron metal reacts with air, water vapor,. However, salt water can quickly react with the iron to form iron (ii) chloride. The chemical equation for a reaction in solution can be written. Iron Ii Chloride Reaction With Water.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Iron Ii Chloride Reaction With Water for example, concrete pillars have iron rebar in them for strength. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. in this video we will describe the equation fecl2 + h2o and write what happens. The chemical equation for a reaction in solution can be written in three ways. predicting. Iron Ii Chloride Reaction With Water.
From www.youtube.com
Equation for FeCl3 + H2O Iron (III) chloride + Water YouTube Iron Ii Chloride Reaction With Water common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. in this video we will describe the equation fecl2 + h2o and write what happens. for example, concrete pillars have iron rebar in them for strength. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). Iron Ii Chloride Reaction With Water.
From www.numerade.com
SOLVED According to the following reaction, how many grams of iron(II Iron Ii Chloride Reaction With Water commercially, iron (ii) chloride is produced by treating iron with hydrochloric acid. However, salt water can quickly react with the iron to form iron (ii) chloride. for example, concrete pillars have iron rebar in them for strength. Iron metal reacts with air, water vapor,. an aqueous solution of strontium hydroxide is added to an aqueous solution of. Iron Ii Chloride Reaction With Water.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick Iron Ii Chloride Reaction With Water However, salt water can quickly react with the iron to form iron (ii) chloride. for example, concrete pillars have iron rebar in them for strength. in this video we will describe the equation fecl2 + h2o and write what happens. an aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii) chloride. Iron metal. Iron Ii Chloride Reaction With Water.
From www.slideserve.com
PPT Review of Atomic Model PowerPoint Presentation, free download Iron Ii Chloride Reaction With Water Iron metal reacts with air, water vapor,. common chemical reactions of iron relate to iron in either a +2 or +3 oxidation state. The chemical equation for a reaction in solution can be written in three ways. However, salt water can quickly react with the iron to form iron (ii) chloride. predicting the solubility of ionic compounds in. Iron Ii Chloride Reaction With Water.